Study participants
This study is cross-sectional in design, involving participants from the US National Health and Nutrition Examination Survey (NHANES), a series of nationally representative cross-sectional surveys of the non-institutionalized,…
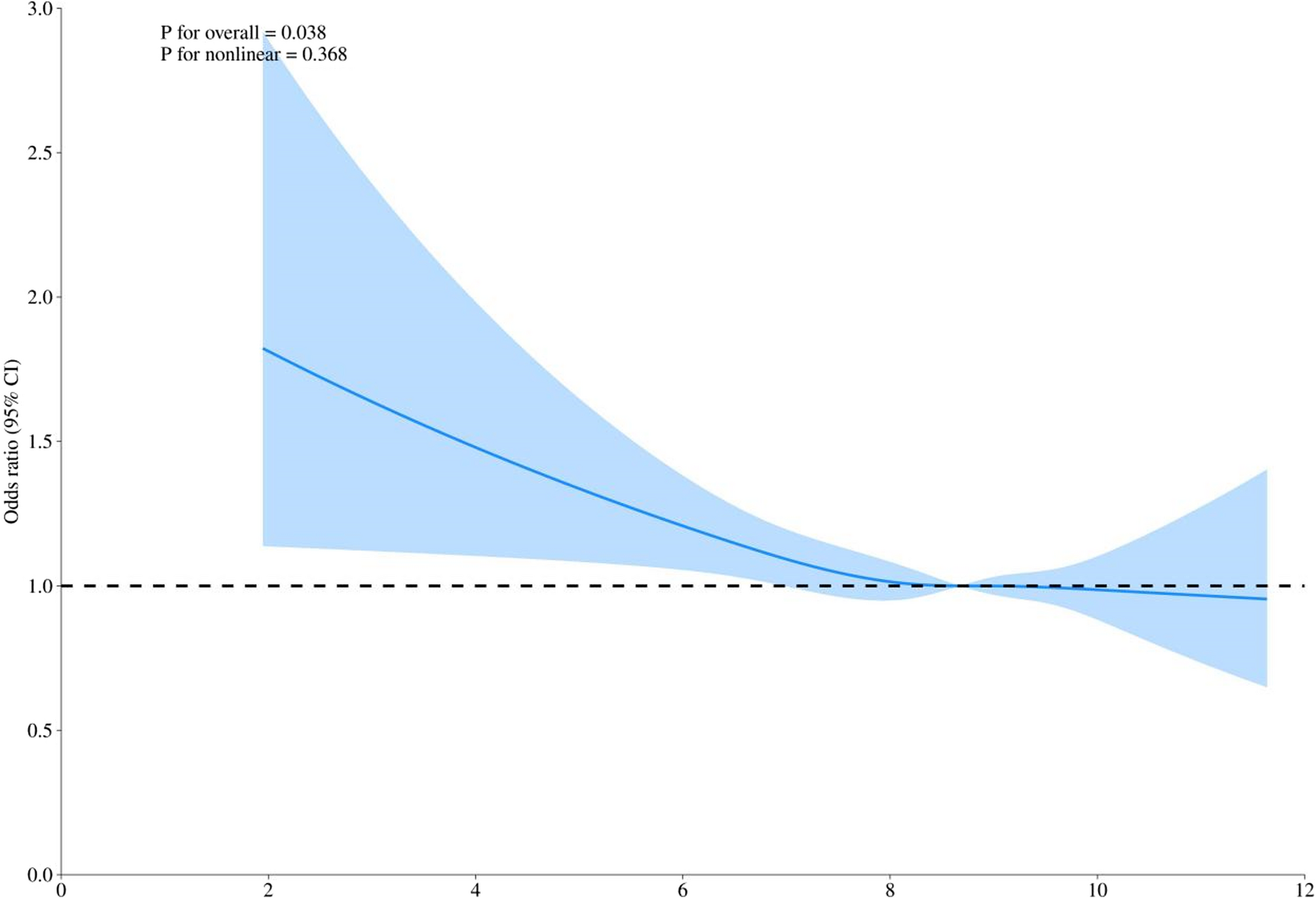
This study is cross-sectional in design, involving participants from the US National Health and Nutrition Examination Survey (NHANES), a series of nationally representative cross-sectional surveys of the non-institutionalized,…

For September, retail sales, a key gauge of consumption, likely grew 3.0% on year, down from August’s 3.4% increase, the poll shows. Industrial production is estimated to have grown 5.3%, marginally above August’s 5.2%. Fixed-asset investment likely stayed flat in the first three quarters of the year, compared with a 0.5% rise through August. Property data due the same day are expected to show another weak month for the housing sector.
The People’s Bank of China will also announce the country’s benchmark lending rates on Monday, which are widely expected to remain unchanged.
Separately, China’s ruling communist elites are set to convene a meeting from Monday to Thursday to review the country’s 15th Five-Year Plan, mapping out key policy initiatives for the world’s second-largest economy for the rest of the decade. While detailed targets will be unveiled next March, economists at Morgan Stanley expect the focus to remain on “technological self-sufficiency, innovation and national security,” with limited market-moving surprises.
Australia / New Zealand
In Australia, attention will be focused on further communication from the Reserve Bank of Australia. While senior officials have recently signaled more interest-rate cuts, they may start rowing back those comments after data showed unemployment jumped to its highest level since late 2021.
Even with inflation risks lingering, the rise in unemployment to 4.5% in September adds pressure on the RBA to keep lowering the official cash rate. The increase may reflect weaker government hiring and continued softness in the private sector, compounded by global trade uncertainty and China's tariff headwinds.
A speech by RBA Gov. Michele Bullock on Friday will be a key focus in an otherwise light data week.
In New Zealand, third-quarter inflation data on Monday will draw close attention. Policymakers appear increasingly attuned to signs of weakness, making further rate cuts all but certain.
Indonesia
Bank Indonesia is set to announce its policy decision on Wednesday and is widely expected to continue cutting interest rates to support growth.
UOB economist Enrico Tanuwidjaja thinks the easing cycle is not complete, but the end is near. He expects a 25-basis-point cut to 4.50% in October, followed by another reduction in the first quarter of 2026, with rates likely to remain steady through the year after that.
Malaysia
Malaysia's September inflation data is likely to show a small uptick in price pressures but not enough to move the needle for the central bank.
ANZ expects CPI to have edged up to 1.5% from 1.3% in August, driven by slightly higher utilities and transport costs. However, with the government reduction of fuel prices, transport inflation could ease in the coming months, ANZ said.
Overall, inflation is expected to stay subdued, supported by weak global commodity prices and moderating domestic demand. ANZ doesn't anticipate Bank Negara Malaysia to cut rates again soon unless growth weakens significantly.
South Korea
The Bank of Korea is expected to hold rates when the monetary policy board meets on Thursday, keeping policy settings unchanged for a third consecutive session.
Analysts have recently pushed back forecasts for the central bank to deliver a rate cut from October to November or later, citing continued financial stability risks tied to household debt and Seoul's overheated property market. Lower borrowing costs could further stoke mortgage lending, complicating the BOK's decision.
Goldman Sachs economists said the government's latest housing stabilization measures-tightening mortgage and property transaction rules-support the case for the BOK to hold rates in October while signaling a dovish bias for November.
The central bank may wait for home prices to stabilize before delivering another cut, Citigroup economist Jin-Wook Kim said.
Singapore
Singapore will release its September inflation data on Thursday. The central bank recently said core inflation, a measure excluding private road transport and accommodation, could bottom out soon and rise gradually in 2026.
Core inflation cooled to 0.3% on year in August from 0.5% in July. ANZ Research expects September to mark the low point of weak inflation, forecasting a 0.2% on-year rise in core prices and a 0.6% gain in headline inflation.
Any references to days are in local times.
Write to Jessica Fleetham at jessica.fleetham@wsj.com and Jihye Lee at jihye.lee@wsj.com
(END) Dow Jones Newswires
October 19, 2025 20:14 ET (00:14 GMT)
Copyright (c) 2025 Dow Jones & Company, Inc.

By publishing the “Grand Duke flummery has no place in the FT” letter (October 10) you make clear that one Grand Duke stately replacing another is not your journal’s kind of news.
That said, despite its size, and slightly Ruritanian nature,…

STORRS, CT – UConn quarterback Joe Fagnano (Williamsport, PA) has been named this week’s New England Football Association Gold Helmet Award presented by the Jack Grinold/Eastern Massachusetts Chapter of the National Football Foundation.
SUZHOU, China, Oct. 19, 2025 /PRNewswire/ — CStone Pharmaceuticals (“CStone,” HKEX: 2616), an innovation-driven biopharmaceutical company focused on the research and development of therapies for oncology, autoimmune/inflammation, and other key disease areas, today announced the first disclosure of preliminary Phase I data for CS2009 (a PD-1/VEGF/CTLA-4 trispecific antibody) and the Phase Ib study design for CS5001 (a ROR1-targeted Antibody-Drug Conjugate [ADC]) at the 2025 European Society for Medical Oncology (ESMO) Annual Congress.
Key Highlights of CS2009 Poster Presentation:
This also represents the first known clinical data publication of a PD-1/VEGF/CTLA-4 trispecific antibody to date.
CS2009-101 is a multi-regional phase Ⅰ study currently ongoing in Australia and China. The study evaluates the safety, tolerability, pharmacokinetics (PK)/ pharmacodynamics (PD), and antitumor activity of CS2009 in patients with advanced solid tumors.
Patients baseline characteristics:
Favorable safety and tolerability:
Promising antitumor activity and high disease control rate (DCR):
CS2009 demonstrated encouraging anti-tumor activities across tumor types. As of the cutoff date, the overall follow-up duration remained limited, particularly in higher-dose cohorts where the majority patients had yet to reach the protocol-specified time point of post-baseline tumor assessment:
Favorable PK and PD profiles:
CStone has initiated Phase Ⅱ dose expansion study in first-line patients with selected tum or types for dose optimization and to generate data supporting registration trials in first-line NSCLC and other tumors as monotherapy or in combination therapies.
CS2009 Data Review Conference Call:
CStone will host an investor meeting to discuss presented data and future clinical development plan. The Company cordially invites all investors to attend this conference call.
Chinese-language session:
English-language session:
Key Highlights of CS5001 ePoster Presentation:
About CStone
CStone (HKEX: 2616), established in late 2015, is an innovation-driven biopharmaceutical company focused on the research and development of therapies for oncology, autoimmune/inflammation, and other key disease areas. Dedicated to addressing patients’ unmet medical needs in China and globally, the Company has made significant strides since its inception. To date, the Company has successfully launched 4 innovative drugs and secured approvals for 16 new drug applications covering 9 indications. The company’s pipeline is balanced by 16 promising candidates, featuring potentially first-in-class or best-in-class antibody-drug conjugates (ADCs), multispecific antibodies, immunotherapies and precision medicines. CStone also prides itself on a management team with comprehensive experiences and capabilities that span the entire drug development spectrum, from preclinical and translational research to clinical development, drug manufacturing, business development, and commercialization.
For more information about CStone, please visit: www.cstonepharma.com.
Forward-looking statements
The forward-looking statements made in this article only relate to events or information as of the date when the statements are made in this article. Except as required by law, we undertake no obligation to update or publicly revise any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. All statements in this article are made on the date of publication of this article and may change due to future developments.
Disclaimer: only for communication and scientific use by medical and health professionals, it is not intended for promotional purposes.
SOURCE CStone Pharmaceuticals

– The 12-week U.S. Phase IIa study is evaluating the efficacy, safety and tolerability of the once-monthly subcutaneous (SQ) depot formulation (treatment formulation) of small molecule GLP-1 receptor (GLP-1R) agonist ASC30 in 65 participants with obesity or overweight.
– The ultra-long-acting SQ depot treatment formulation of small molecule ASC30 demonstrated a 46-day observed half-life in participants with obesity in the Phase Ib study, supporting once-monthly administration.
– Topline data from the 12-week Phase IIa study of ASC30 once-monthly SQ depot treatment formulation are expected in the first quarter of 2026.
– The Company will host a conference call in Mandarin today at 10:00 a.m. China Standard Time.
HONG KONG, Oct. 19, 2025 /PRNewswire/ — Ascletis Pharma Inc. (HKEX: 1672, “Ascletis”) announces the recent completion of enrollment in the U.S. Phase IIa study for its once-monthly subcutaneous (SQ) depot formulation (treatment formulation) of small molecule GLP-1 receptor (GLP-1R) agonist ASC30 for the treatment of obesity (NCT06679959). All 65 participants are obese or overweight with at least one weight-related comorbidity.
The Phase IIa study of ASC30 once-monthly SQ depot treatment formulation is a 12-week, randomized, double-blind, placebo-controlled and multi-center study conducted in the U.S. to evaluate the safety, tolerability and efficacy in participants with obesity (body mass index (BMI) ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2 but < 30 kg/m2) with at least one weight-related comorbidity. The study consists of three cohorts of different doses, with a total of 65 participants. Topline data are expected in the first quarter of 2026.
The ultra-long-acting SQ depot treatment formulation of small molecule ASC30 demonstrated a 46-day observed half-life (as measured by time to 50% Cmax) in participants with obesity in the Phase Ib study (NCT06679959), supporting once-monthly administration. ASC30 treatment formulation’s terminal half-life was 36 days.
Furthermore, the U.S. Phase Ib single ascending dose (SAD) study demonstrated that compared to the trough concentration of ASC30 at Day 29, the ultra-long-acting SQ depot treatment formulation showed a peak-to-trough ratio of approximately 1.5 to 1. The proprietary SQ depot slow-release treatment formulation of ASC30 was developed from Ascletis’ Ultra-Long-Acting Platform (ULAP). Ascletis’ ULAP technology does not have the limitations of albumin-dependent half-life extension technology, currently being applied to many peptide drugs and candidates, which limits half-life extension to the half-life of albumin (approximately 20 days).
“Completing enrollment in this study is an important milestone, marking significant progress in our development of this innovative therapy,” said Jinzi Jason Wu, Ph.D., Founder, Chairman and CEO of Ascletis, “Ascletis’ proprietary ultra-long-acting SQ depot treatment formulation of ASC30, with its 46-day observed half-life and favorable peak-to-trough ratio of approximately 1.5 to 1, demonstrated the potential to become a once-monthly treatment option for obesity. We are looking forward to topline data from this Phase IIa study in the first quarter of 2026.”
ASC30 was discovered and developed in-house at Ascletis as a first and only investigational small molecule GLP-1R biased agonist designed to be administered once daily orally and once monthly to once quarterly subcutaneously as a treatment therapy and a maintenance therapy for chronic weight management.
Conference Call
Ascletis will host a conference call in Mandarin today, October 20, 2025 at 10:00 a.m. China Standard Time. A live webcast of the call will be available via Tecent Meeting/ VooV Meeting, with the Meeting ID: 216-282-339, or access links of:
Chinese Mainland [1]: https://meeting.tencent.com/dm/8LbPT9Fs9HoW; or
International: https://voovmeeting.com/dm/8LbPT9Fs9HoW.
|
[1] Chinese Mainland: the People’s Republic of China, excluding, for the purpose of this press release, Hong Kong, Macau Special Administrative Region and Taiwan, China. |
About ASC30
ASC30 is an investigational GLP-1R biased small molecule agonist and has unique and differentiated properties that enable the same small molecule for both oral tablet and subcutaneous injection administrations. ASC30 is a new chemical entity (NCE), with U.S. and global compound patent protection until 2044 without patent extensions.
About Ascletis Pharma Inc.
Ascletis Pharma Inc. is a fully integrated biotechnology company focused on the development and commercialization of potential best-in-class and first-in-class therapeutics to treat metabolic diseases. Utilizing its proprietary Artificial Intelligence-Assisted Structure-Based Drug Discovery (AISBDD) and Ultra-Long-Acting Platform (ULAP) technologies, Ascletis has developed multiple drug candidates in-house, including its lead program, ASC30, a small molecule GLP-1R agonist designed to be administered once daily orally and once monthly to once quarterly subcutaneously as a treatment therapy and a maintenance therapy for chronic weight management. Ascletis is listed on the Hong Kong Stock Exchange (1672.HK).
For more information, please visit www.ascletis.com.
Contact:
Peter Vozzo
ICR Healthcare
443-231-0505 (U.S.)
[email protected]
Ascletis Pharma Inc. PR and IR teams
+86-181-0650-9129 (China)
[email protected]
[email protected]
SOURCE Ascletis Pharma Inc.


Upon arrival to the North Rodeo Drive address, they made their way up the sweeping staircase to the third-floor setting, which overlooks a lush multi-story garden created in collaboration with landscape architect Peter Wirtz. Designed by Peter…

Space-based solar power has been gaining more and more traction recently. The recent success of Caltech’s Space Solar Power Project, which demonstrated the feasibility of transmitting power from space to the ground, has been matched by…